
About biopexFill®
Established in 2020, Glopex Co., Ltd is fast growing in the global aesthetic market, aiming to be a developer and manufacturer of biomedical and biopharmaceutical products and services in the global market.
Glopex®‘s main business area not only focuses on the biomedical field, but we are also setting our first steps to advance in medicine-based biotechnologies to improve overall global health.
With continuous in-house research and development, we plan to develop different types of advanced hyaluronic acid gels, botulinum toxins, and other biomedical and biopharmaceutical products with our exclusive technologies.
Glopex® has innovated its biopexFill® HA gel lines that consist of various injectable cross-linked hyaluronic acid gels with products that come in 1.0 mL and 10 mL syringes in addition to 60 mL vials with distinctive uses for either face or body and come in different properties and densities to add volume and fullness to the skin.
The different processes used in our manufacture and formulation generate our products with special cohesivity, plasticity, and viscoelasticity properties that make biopexFill® a unique performance hyaluronic acid gels that shows the most natural and longest-lasting results.
Glopex® stands out for its customer services, where customers can enjoy membership benefits and awards as biopexFill® club members.
biopexFill® Technology
Technology
High-Density Hyaluronic Acid Gel
biopexFill® is manufactured of high-density HA gel using a unique Cross-Linking Technology to create a higher viscoelasticity gel that is safe to use. The benefits of biopexFill® high-density HA are:

High Volumizing and Lifting Capacity
biopexFill®‘s gel has a high elasticity in the market. G′ representing elasticity is an indicator showing the results of the gel’s volumizing effect and lifting effect. biopexFill® can immediately give the desired volume effect even when used in small amounts.
Safety and Purification
• Modification Degree (MoD)
MoD is the Modification Degree of the hyaluronic acid molecule by bound BDDE. The lower the cross-linking rate, the more similar it is to the natural hyaluronic acid properties, which means it is a safe gel. The graphs shows that the biopexFill® cross-linking rate is remarkably lower than other tested brands.
• Removal of Residual BDDE
If the remaining BDDE used in cross-linking is not entirely removed, it can trigger an allergic reaction in the human body.
The process of removing residual BDDE generated by cross-linking through the refinement method of biopexFill® has improved the product’s safety.
For high refining efficiency, biopexFill® crushes the HA gel before purification and exposes it directly to the “wash buffer” so that there is no BDDE in the purified HA gel.
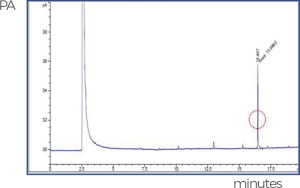
[Standard BDDE Detection]

[biopexFill® BDDE Detection]
